electron configuration for calcium|What is the electron configuration of calcium? : Baguio Learn how to write the electron configuration for calcium using the period table or an electron configuration chart. See the video and the step-by-step tutorial for writing the configuration notation. [WATCH VIDEO LEAKED] Meia Cassandra Viral Video Leaked; scandal explained by videoviral, released 02 July 2024
PH0 · Write the electronic configuration for calcium.
PH1 · What is the electron configuration of calcium?
PH2 · Electron configuration of calcium
PH3 · Electron Configuration for Calcium (Ca, Ca2+ ion)
PH4 · Electron Configuration for Calcium (Ca, Ca2+ ion)
PH5 · Electron Configuration for Calcium (Ca)
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Chemistry of Calcium (Z=20)
PH8 · Calcium Electron Configuration (Ca) with Orbital Diagram
PH9 · Calcium Electron Configuration
PH10 · Calcium
PH11 · 3.1: Electron Configurations
Race to Riches Scratchcard This scratchcard can be used by taking it to the Ice Caves Kiosk, and rubbing off 6 of the 9 circles. 3 of a kind wins a prize! Add To List.. Find At. Item Info. Item ID. 8903. Rarity. r101 (special) Weight. 1 lbs. Est. Val. 550 NP. Category. Special. Status. active. itemdb ID. 35. Color Palette. #FCCD14. vibrant. # .
electron configuration for calcium*******Learn how to write the electron configuration for calcium using the period table or an electron configuration chart. See the video and the step-by-step tutorial for writing the configuration notation.
Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s .
Therefore the Chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 .
119 rows — Mar 23, 2023 — Find the electron configuration of calcium (Ca) .Learn how to write the electron configuration of calcium, an alkaline earth metal with 20 electrons, using Kerner's rule and noble gas reference. Find out the properties, isotopes .Nob 18, 2013 — A step-by-step description of how to write the electron configuration for Calcium (Ca). In order to write the Ca electron configuration we first need to know the number of electrons for the.Ene 16, 2016 — Calcium has an atomic number of 20. Simply use this information to obtain its electronic configuration. Thus, #Ca: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2# or simply #Ca: .
Ene 21, 2021 — Ground State Electron Configuration for Calcium. While writing the configuration we put all 20 electrons in orbitals around the calcium’s nucleus atom. So Ca electron configuration is 1s2 2s2 2p6 .Thus, potassium has an electron configuration of [Ar]4s 1. Hence, potassium corresponds to Li and Na in its valence shell configuration. The next electron is added to complete the 4s subshell and calcium has an .Hun 30, 2023 — Calcium is the 20th element in the periodic table.
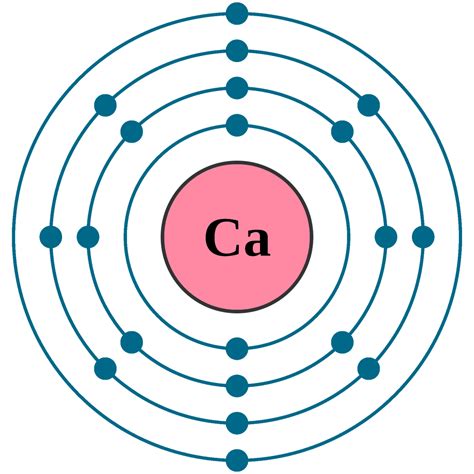
Electron configuration of Calcium is [Ar] 4s2. Possible oxidation states are +2.
Hun 16, 2024 — The electronic configuration of calcium is 1s2 2s2 2p6 3s2 3p6 4s2. In calcium hydroxide (Ca(OH)2), calcium loses its two outer electrons to form Ca2+ ion, which has the electronic configuration .Ene 16, 2016 — Ca: [Ar] 4s^2 Calcium has an atomic number of 20. Simply use this information to obtain its electronic configuration. Thus, Ca: 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 or simply Ca: [Ar] 4s^2.Mar 20, 2023 — The arrangement of electrons in cadmium in specific rules in different orbits and orbitals is called the electron configuration of cadmium. The electron configuration of cadmium is 4d 10 5s 2, if the .Mar 18, 2023 — Gallium excited state electron configuration. Atoms can jump from one orbital to another orbital in the excited state. This is called quantum jump. The ground state electron configuration of gallium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 1. In the gallium ground-state electron configuration, the last electron of the 4p orbital is located in .Question: Give the full electron configuration for calcium (Ca). electron configuration: BI V x2x2 Show transcribed image text There’s just one step to solve this.May 21, 2024 — For calcium, the electron configuration is: 1s2 2s2 2p6 3s2 3p6 4s2. Using the noble gas argon (1s2 2s2 2p6 3s2 3p6) as the starting point, the noble gas notation for calcium is [Ar] 4s2.Dis 8, 2018 — The electron configuration for a calcium atom is represented as 1s2, 2s2, 2p6, 3s2, 3p6, 4s2. This means that in the first energy level (n=1), there are two electrons in the s sublevel (1s2). In the second energy level (n=2), there are two electrons in the s sublevel (2s2) and six electrons in the p sublevel (2p6). In the third energy level (n .Ene 30, 2023 — The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. . For instance, the ground state electronic configuration of calcium (Z=20) is 1s 2 2s 2 2p 6 3s 2 3p 6 4s .Study with Quizlet and memorize flashcards containing terms like Listed below is an incorrect electron configuration for calcium. Identify what is wrong in the electron configuration. Ca = 1s11s22s22p63s23p64s2, How many d electrons are found in cobalt?, How many valence electrons are present in sulfur? and more.

Mar 17, 2023 — The electron configuration of an element with an atomic number greater than 18 cannot be properly determined according to the Bohr atomic model. The electron configuration of all the elements can be done through orbital diagrams. Electron configuration of potassium through orbital.Nob 18, 2013 — A step-by-step description of how to write the electron configuration for Calcium (Ca). In order to write the Ca electron configuration we first need to kno.Therefore, the electronic configuration of sulfur can be written as 1s 2 2s 2 2p 6 3s 2 3p 4. The electronic configuration of elements can also be written with the help of noble gases. These noble gases have .Hun 9, 2024 — The electron configuration for neutral calcium is 1s2 2s2 2p6 3s2 3p6 4s2. When calcium forms a +2 ion, it loses the two electrons in the 4s subshell, so the electron configuration becomes 1s2 2s2 .electron configuration for calciumOkt 12, 2023 — The electron configuration of Calcium in terms of the shell or orbit is [2, 8, 8, 2]. The ground-state electron configuration of the Calcium (Ca) atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. And for the excited state, it is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 4p 1. The shorthand electron configuration for Calcium is [Ar] 4s 2.
What is the electron configuration of calcium? Okt 12, 2023 — The electron configuration of Calcium in terms of the shell or orbit is [2, 8, 8, 2]. The ground-state electron configuration of the Calcium (Ca) atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. And for the excited state, it is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 4p 1. The shorthand electron configuration for Calcium is [Ar] 4s 2.Mar 18, 2023 — Therefore, the electron configuration of nickel(Ni*) in an excited state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d xy 2 3d yz 2 3d zx 2 3d x 2-y 2 1 3d z 2 1 4s 1 4p x 1. The valency of the element is determined by electron configuration in the excited state. Here, nickel has four unpaired electrons. So, in this case the valency of nickel is 4 .
Hun 16, 2024 — The electron configuration for a calcium atom with atomic number 20 is 1s2 2s2 2p6 3s2 3p6 4s2. This can be represented using the noble gas shorthand notation as [Ar] 4s2, where [Ar] represents .Mar 21, 2023 — When writing an electron configuration, you have to write serially. Cesium ion(Cs +) electron configuration. The ground-state electron configuration of cesium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 1. This electron configuration shows that the last shell of cesium has only an electron. Therefore, the valence .
What is the correct electron configuration of the ion formed by sulfur? What is the mass number of calcium? Why is calcium placed in 2 group although it has completely filled orbitals? What is the electron configuration for an aluminum ion, Al3+? What do the electron configurations for all the group 18 noble gases have in common?
Typically I’d use it for exactly $5 items or a little higher to either get the item free or pay a small amount for that item. Today I received a letter from PRA law firm saying I need to pay $800 for restitution component plus an additional $200 for civil penalty, totaling $1000 that I have to pay in 20 days. I’m freaking out it’s been 4 .
electron configuration for calcium|What is the electron configuration of calcium?